You are invited to join a team of passionate professionals
With 7 years of experience, our research-driven company is dedicated to enhancing the health of millions by supporting quality medicine manufacturing and testing. By joining Indivirtus at one of our locations, you will be part of an organization that fosters a high-performing culture. We prioritize continuous improvement and collaboration in a supportive and transparent environment.
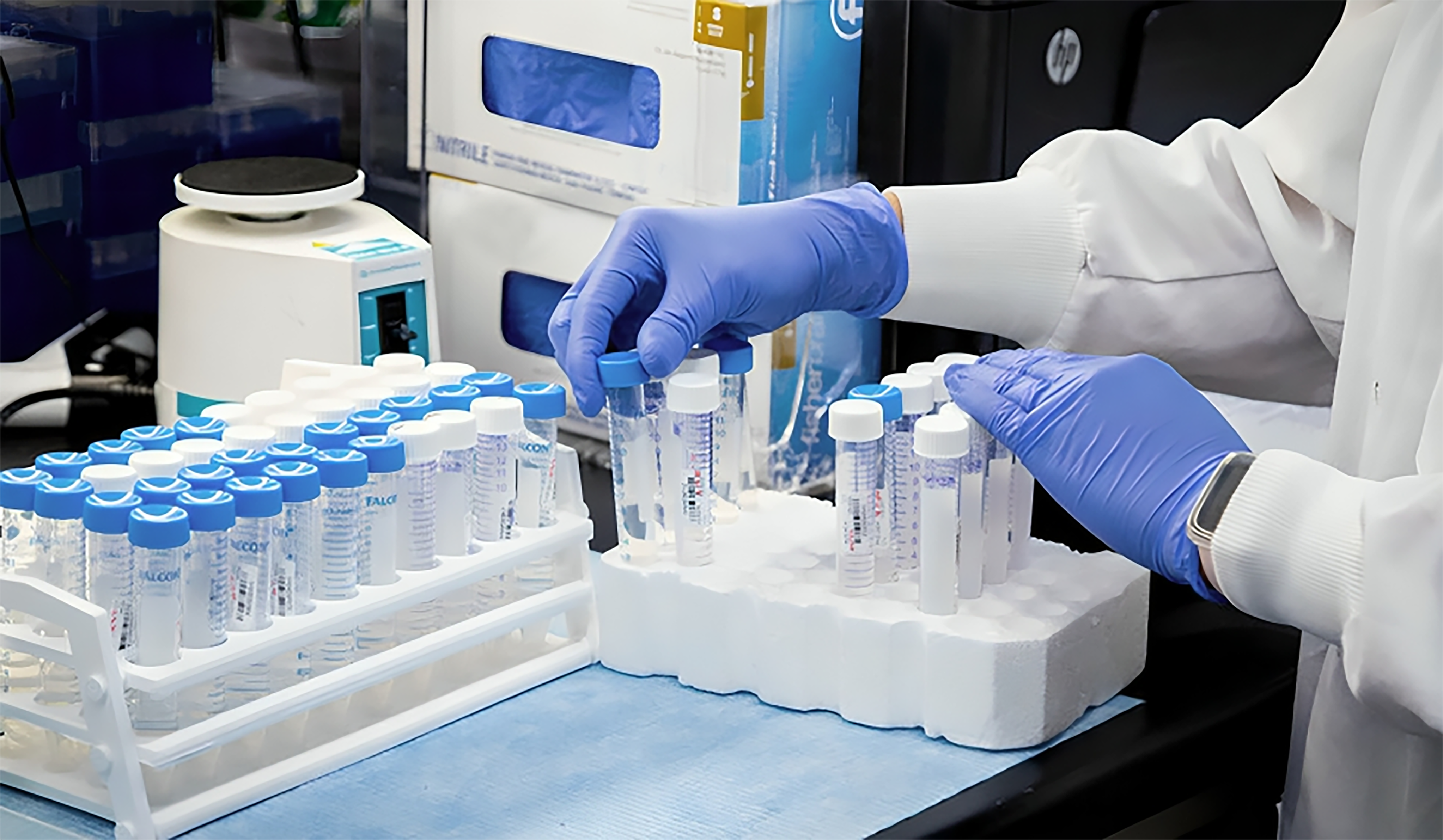
Role: Sample Processing for MD, MV and Biostudy, LCMS Handling
Experience: 1 to 5 Years
Salary: ₹2,50,000 to ₹7,00,000
Location: Mohali
Posted On: 12-Nov-2025
Role: Site Management, Subject Safety, Data Integrity and Regulatory Compliance
Experience: 8 to 20 Years
Salary: ₹12,00,000 to ₹39,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Site Management, Backup Leadership, Subject Safety, Data Integrity and Regulatory Compliance
Experience: 7 to 10 Years
Salary: ₹9,00,000 to ₹12,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Primary point of contact with sponsor/CRO for communication, Protocol Adherence, Subject Safety, informed consent, Data Integrity & Regulatory Compliance
Experience: 5 to 10 Years
Salary: ₹5,50,000 to ₹6,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Protocol execution, Regulatory & Ethics Compliance, Subject Management, Data & Documentation, Cross-functional Coordination
Experience: 2 to 4 Years
Salary: ₹2,50,000 to ₹4,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Assist in protocol development, ensure proper handling, storage & dispensing of investigational products, maintain documentation for audits, safety & compliance
Experience: 2 to 6 Years
Salary: ₹3,00,000 to ₹6,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Protocol, ICF & CRF Writing and approvals
Experience: 4 to 8 Years
Salary: ₹8,50,000 to ₹17,50,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Study report preparation, submission, archival, query handling
Experience: 2 to 6 Years
Salary: ₹5,80,000 to ₹8,80,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Raw data, Report adherence to protocol & regulatory standards, query handling
Experience: 4 to 8 Years
Salary: ₹7,50,000 to ₹9,70,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Protocol Compliance, Data Integrity, Training & Oversight, Documentation Review, Audit & Monitoring
Experience: 2 to 8 Years
Salary: ₹6,50,000 to ₹25,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Protocol Compliance, Data Integrity, Training, Oversight, Documentation Review, Audit & Monitoring, Regulatory Liaison, Risk Management
Experience: 1 to 3 Years
Salary: ₹1,80,000 to ₹4,50,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Document Control & Archiving, Regulatory Compliance, Retention & Retrieval, Digital Archiving, Audit Support
Experience: 1 to 3 Years
Salary: ₹1,80,000 to ₹2,50,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025
Role: Equipment Maintenance & Calibration, Software & IT Support, Regulatory Compliance, Operational Support
Experience: 3 to 5 Years
Salary: ₹3,00,000 to ₹5,00,000
Location: Bhuvneshwar
Posted On: 12-Nov-2025